Declaring cosmetics in Vietnam is a legal requirement ensuring product safety and quality. This process protects consumers and builds trust in cosmetic businesses.
What is Cosmetic Product Declaration?
It is a mandatory procedure for organizations or individuals to register their products with the regulatory authority before entering the Vietnamese market. Upon approval, a receipt number is issued, confirming the product’s legal circulation.
Key Regulations
According to Circular 06/2011/TT-BYT:
- All imported and domestically produced cosmetics must have a receipt number before distribution.
- Products may undergo post-market inspections.
- Businesses are responsible for product safety, quality, and compliance.
Required Documents
- Business Registration Certificate: Confirms legal operations in cosmetics.
- Authorization Letter: Required for importers/distributors; must be consularly legalized.
- Declaration Form (2 Copies): Includes product details, manufacturer/importer information, and legal compliance commitment.
- Certificate of Free Sale (CFS): Valid, legalized proof from the manufacturing/exporting country for imported products.
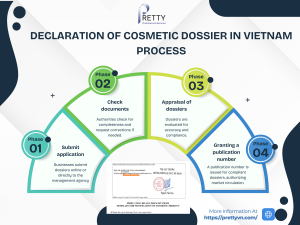
Declaration Process
- Submit the dossier in person or online.
- Authorities review for completeness and validity.
- Approved dossiers receive a receipt number, authorizing market entry.
Contact :